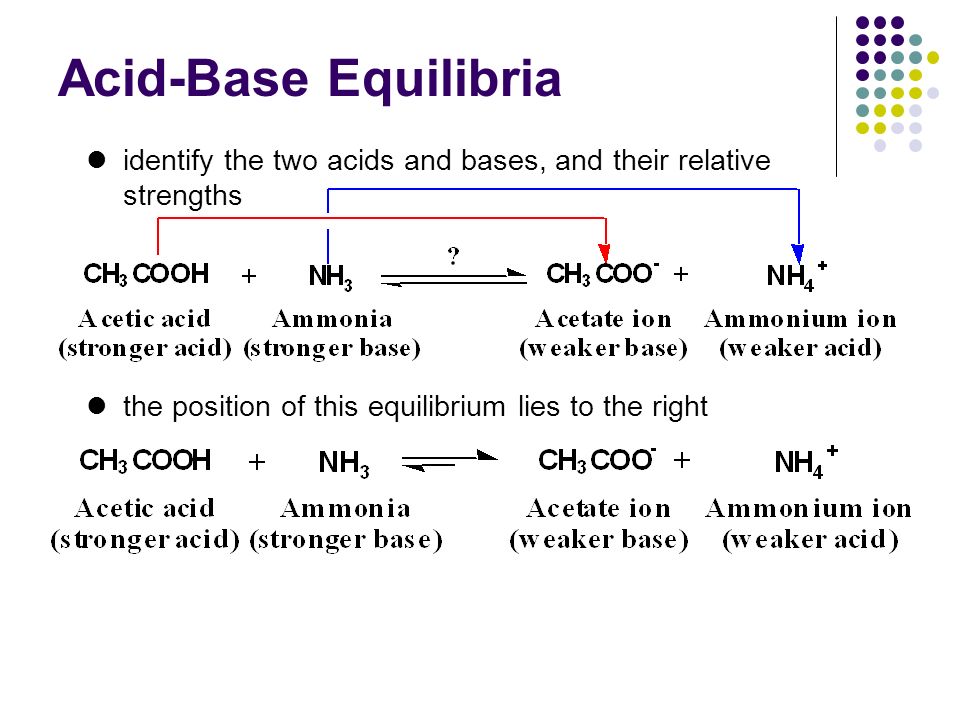
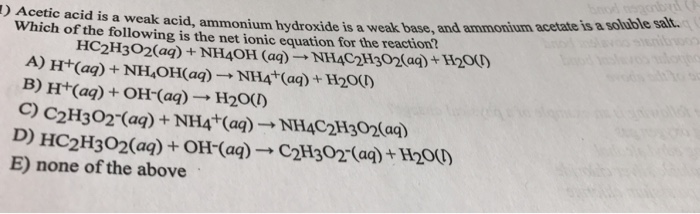pka of acetic acid and pKb of ammonium hydroxide are 4.76 and 4.75 respectively. Calculate the pH of ammonium acetate solution.

Spontaneity of the acid–base reaction between acetic acid and ammonia... | Download Scientific Diagram

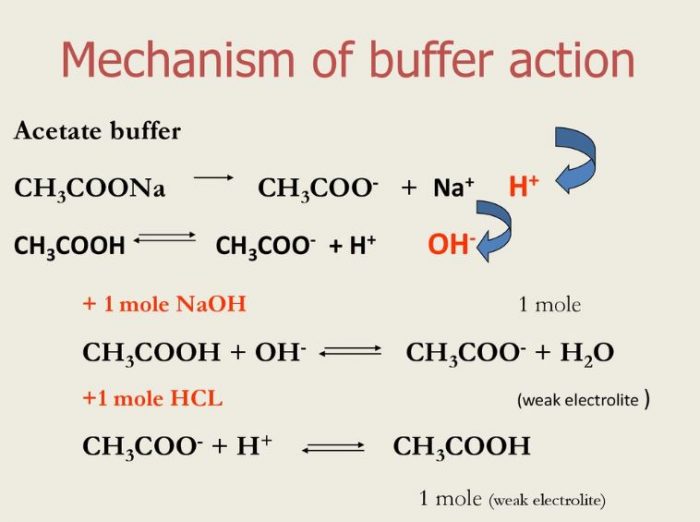
inorganic chemistry - Is the salt of a weak acid and a weak base also a weak electrolyte? - Chemistry Stack Exchange
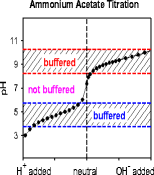
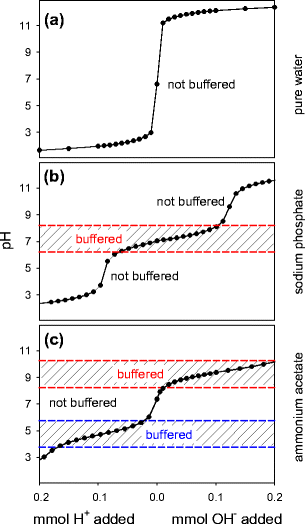
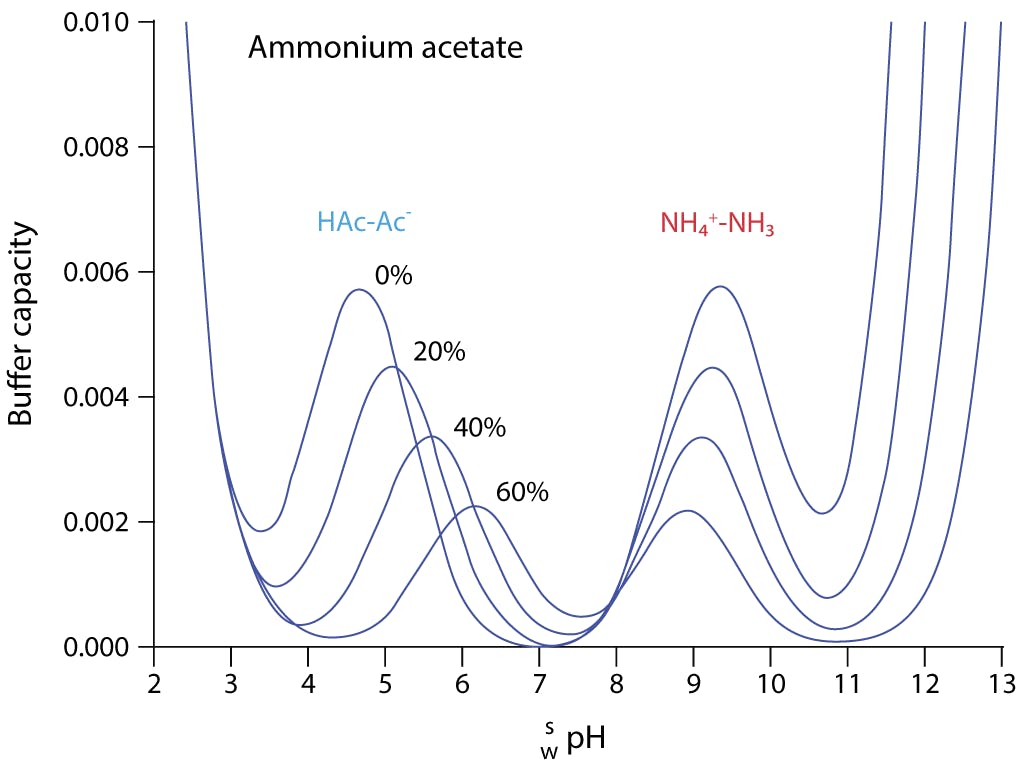
Addressing a Common Misconception: Ammonium Acetate as Neutral pH “Buffer” for Native Electrospray Mass Spectrometry | SpringerLink
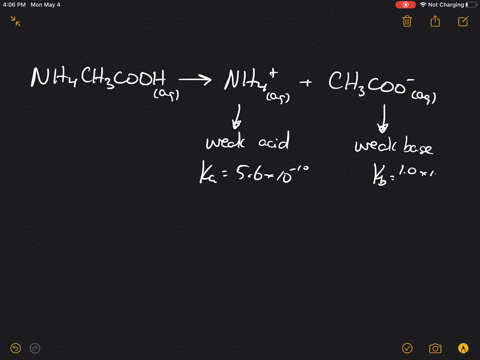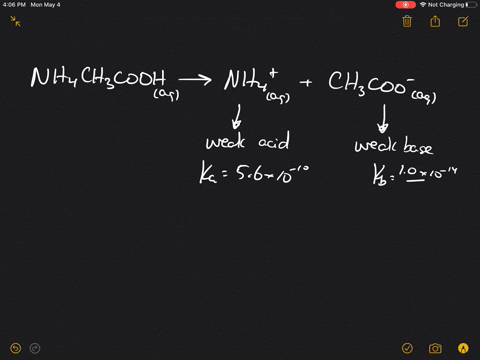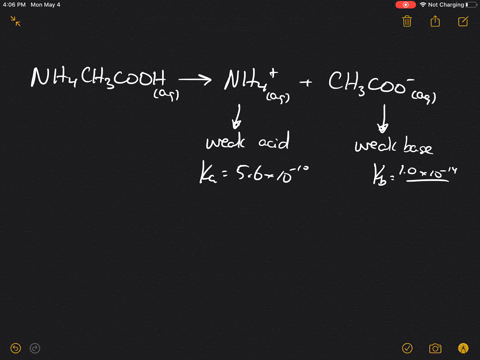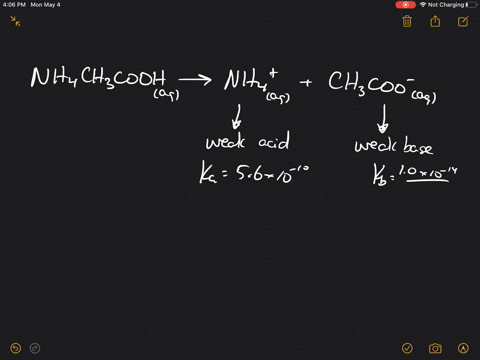
SOLVED:Which of the following salts produces an acidic solution in water: ammonium acetate, ammonium nitrate, or sodium formate?